Optimal timing of antenatal corticosteroids to improve outcomes in preterm birth
This QI project aimed to increase the proportion of women at less than 34 + 0 weeks’ gestation with threatened preterm labour receiving a full course of antenatal corticosteroids within one week prior to delivery to 95% or greater by March 2023.
Publication date: 12 October 2023
Authors
Health Innovation Oxford and Thames Valley (formerly the Oxford Academic Health Science Network – Oxford AHSN)
Eileen Dudley, Senior Programme Lead & MatNeoSIP Lead Patient Safety Collaborative, Health Innovation Oxford and Thames Valley
Michelle East, Lead Midwife, Governance & Quality, Bucks Healthcare & MatNeoSIP Regional Midwifery Clinical Improvement Lead
Background and context
The Maternal and Neonatal Safety Improvement Programme (MatNeoSIP) was established to support England’s national ambition to reduce rates of maternal and neonatal deaths, stillbirths, and brain injuries by 50%, by 2025. MatNeoSIP has focussed on seven evidence-based interventions, to support the goal of optimising care of the preterm infant. This Quality Improvement Project (QIP) focusses on one of those interventions – the correct and timely administration of antenatal corticosteroids within the 7 days prior to anticipated preterm birth.
Project aim
To increase the proportion of women at less than 34 + 0 weeks’ gestation with threatened preterm labour receiving a full course of antenatal corticosteroids within one week prior to delivery to 95% or greater by March 2023.
Method and data collection
The NNAP data available at the outset of this QIP reflects whether mothers were given steroids during the antenatal period, but not whether the course was completed, i.e. two separate doses at least 12h apart, or in the 7-days prior to the birth target timeline. Therefore, a data gathering phase occurred between April 2021 & November 2022, to retrieve the required information.
A proforma was designed to enable a more consistent approach to data collection and refined using PDSA improvement cycles.
Over the above period, there were 83 births at < 34 weeks’ gestation. 95% of women received steroids during the antenatal period. 73% of these women received a complete course and 48% had steroids within 7 days of birth.
Steroids prior to planned iatrogenic preterm birth accounted for approximately 25% of all women eligible for inclusion in the QIP. Surprisingly, repeat courses were unusual.
The reasons for not achieving optimal timing and administration of a full course included rapid progression of labour or birth occurring prior to admission to a maternity unit.
Of those who received a full course of steroids outside the seven days prior to birth, data showed that there were inconsistencies regarding risk assessment across the Oxford AHSN (Thames Valley) region. Whilst data collection was not comprehensive enough to determine the intricacies of decision-making, inconsistencies were identified in the criteria for corticosteroid administration; type of corticosteroid used and route of administration.
Project stakeholders
Our stakeholders were senior healthcare professionals from Trusts within the Thames Valley Region.
A driver diagram (Figure 1) was developed as an output from the stakeholder group discussions, to provide direction for future activity and to define areas of focus.
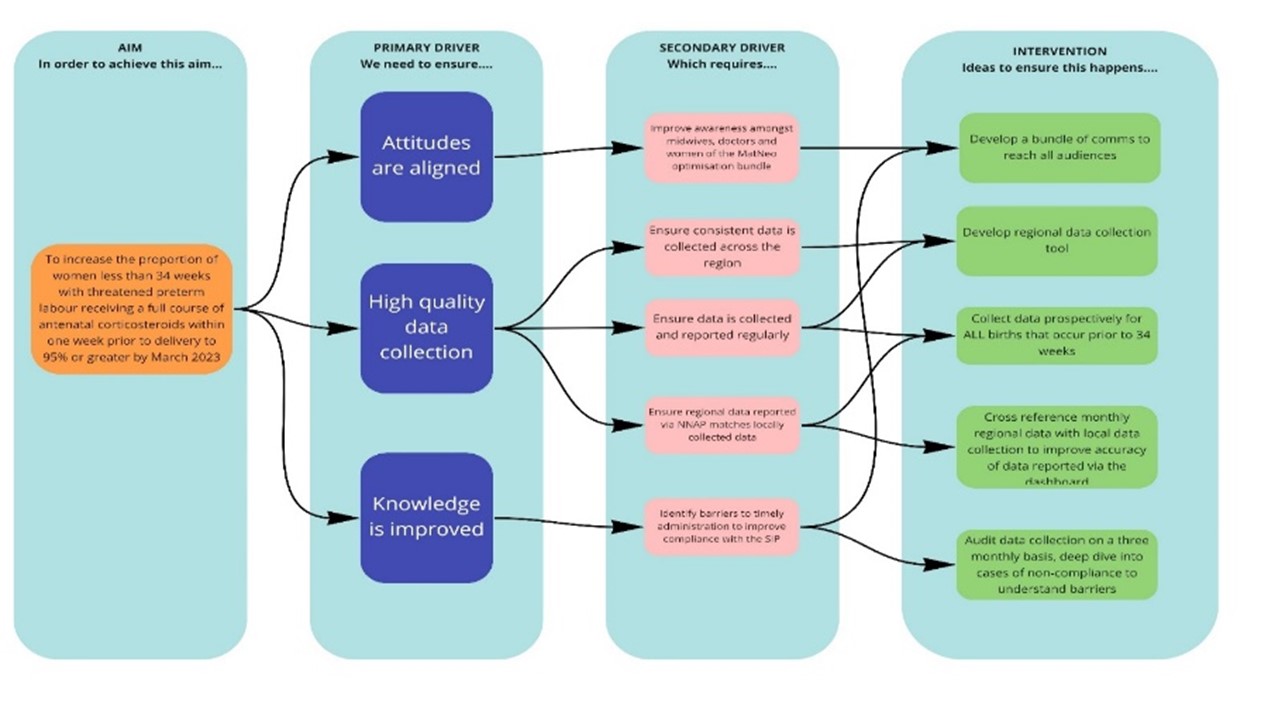
A monthly Task & Finish QI working group comprised of senior obstetric and midwifery leads, and trainees from each of our stakeholder organisations, was established to agree a strategy and maintain momentum. Importantly, each Trust had responsibility to raise awareness and support for the QIP within their own local multi-professional teams.
Areas for improvement
The project group agreed on key areas for focussed improvement:
- Administering antenatal corticosteroids appropriately: Our data indicated that clinical decision making related to optimal timing of steroids was poor and it was often a junior doctor’s decision in a maternity triage. The culture reflected a normalisation in the prescription and administration of antenatal steroids.
- Completing the course: This may not always be possible due to the unpredictability of preterm labour and birth but warrants closer scrutiny
- Improved accuracy of the diagnosis of preterm labour and birth: This is a considerable challenge but will be improved by senior consultation and decision making and the increased use of point of care (POC) tests combined with accurate and detailed history taking.
Our improvement plans
- Development of a Regional Network Guideline to maximise the chance of steroids within 7 days of preterm birth.
- Adoption of the QUiPP App combined with Quantitative Fetal Fibronectin (qfFN) and cervical length measurement.
- Development of an educational resource pack to support education and training for multi-professional teams.
The evidence highlighted the need for a consistent approach to decision-making at network level through the introduction of a regional guideline and a tool to improve specificity and sensitivity, to support better decision making. The British Association of Perinatal Medicine (BAPM) advocates the use of the QUiPP App, combined with quantitative foetal fibronectin (qfFN) and cervical length measurement to achieve this.
Since the data collected locally showed inconsistent use of prediction techniques, development of a regional guideline that advocates the use of the QUiPP App was identified as a potential intervention. This would enable more accurate risk assessment and optimal timing of antenatal corticosteroids, potentially reducing the administration of repeat doses.
Figure 2 – Preterm optimisation dashboard: NHS Futures, accessed 29 August 2023
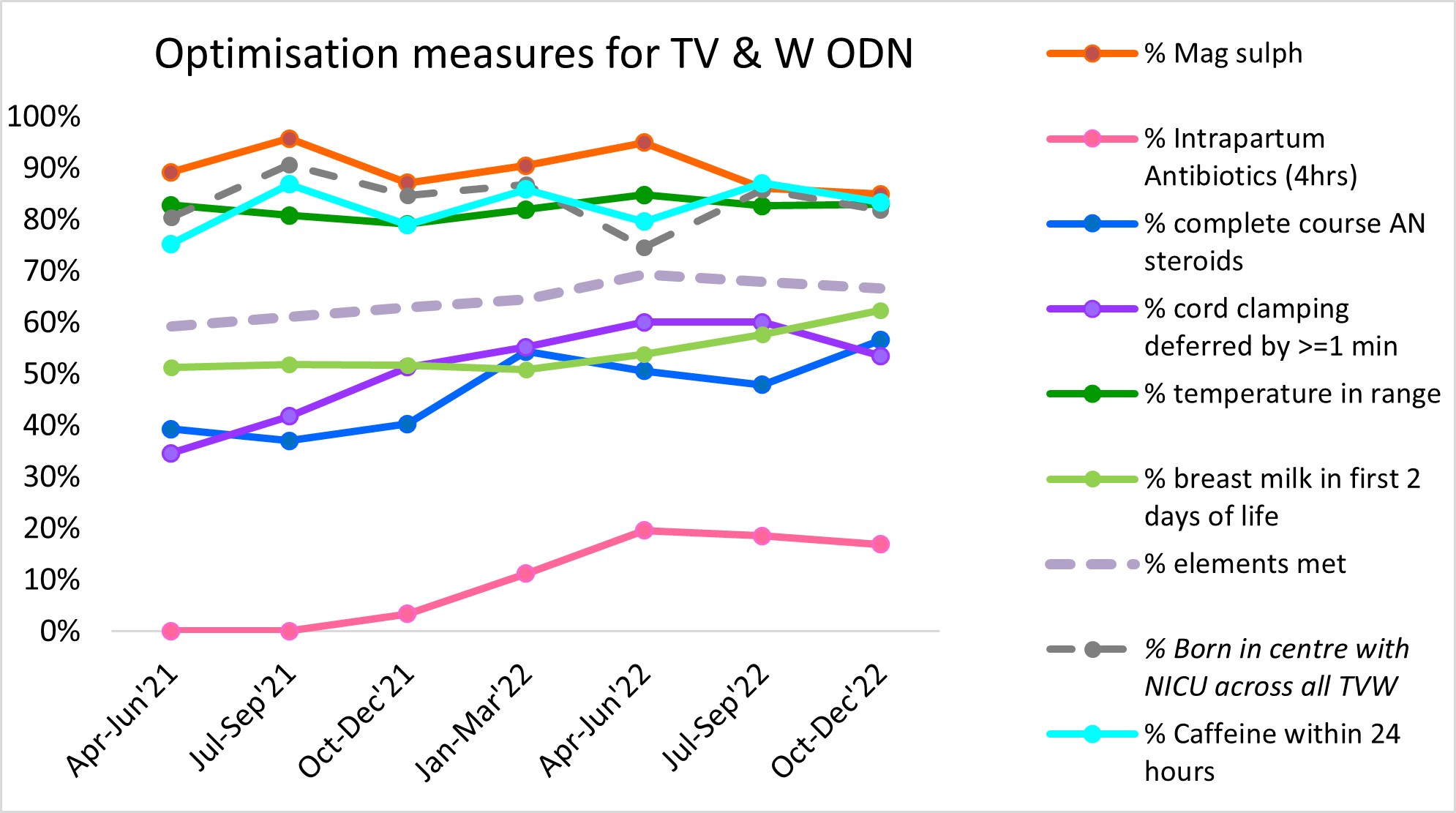
Outcome of the QIP
The above data shows stability or improvement across all elements of the bundle. The focus of this QIP was on the administration of antenatal steroids, and despite the challenges outlined in this document, there has been a steady improvement from April 2021 (39%) to December 2022 (57%). This has provided confidence to the group that if all interventions are embedded the data should continue to show improvement.
Top tips for implementation
- Committed leadership
- Clinical expertise and credibility
- Professional curiosity: know your local and regional baseline data
- Champions who are passionate about the project
- Monthly QI huddles to sustain momentum, share ideas, challenges, and solutions
- Communication bundle for Trusts, which included a formal written report and infographics posters
- Certificates of achievement for members of the project team
Sustaining improvement
Ongoing monitoring of performance will be reported via the optimisation dashboard. This will not only demonstrate performance across the Thames Valley region, but will also enable national comparisons to be made, which will support the possibility of horizon scanning and shared learning. Each Trust will ensure that the optimisation data forms part of the standard reporting within their organisation, to identify where performance levels need attention.
Challenges
- Data collection was challenging in the initial project phase was because the scope was too broad and the retrospective data was very difficult to collect.
- There was a considerable requirement for education and raising awareness of the evidence base underpinning optimal timing of antenatal corticosteroids required for all our healthcare professionals.
- The shift required in mindsets, behaviours and culture within teams should not be underestimated.
- The national shortage of fFn Cassette Kits meant that we were unable to progress with our network adoption of the QUiPP App in the way that we had agreed.
Learning
- This QIP continues to be a brilliant project because of the opportunity it presents to increase knowledge and awareness supporting evidence-based clinical practice and potentially reducing the harm associated with inappropriate use of antenatal corticosteroids. In the future, the project team hopes to extend data collection to those who receive steroids, but do not experience preterm birth, as this is known to increase harm.
- The discovery phase and understanding of local and regional baseline data to inform focussed improvement was a critical success factor for our work. Discussions and challenging conversations during this phase were instrumental in securing engagement and ongoing interest in the QIP.
- Monthly QI huddles were valued as a means of support, information exchange and brainstorming challenges as they arose.
- Accept that this is complex work. It takes time and there will be setbacks and challenges, but incremental progress is brilliant.
Acknowledgements
- Mr Lawrence Impey, Consultant in Obstetrics & Fetal Medicine, Oxford University Hospitals & Clinical Lead, Regional Maternity & Neonatal Clinical Network, Oxford AHSN
- Ms Meena Bhatia, Consultant in Obstetrics & QI Lead Oxford University Hospitals & MatNeoSIP Obstetric Clinical Improvement Lead
- Alison Provins, Public Partner Oxford AHSN
- Oxford University Hospital NHS FT
- Buckinghamshire Healthcare NHS FT
- Royal Berkshire NHS FT
- Frimley Health NHS FT
- Milton Keynes University Hospitals NHS FT
- Service User Voice Lead, South East Perinatal Team
